Chattanooga Intelect Mobile 2 Combo
Free Delivery on orders over £60*
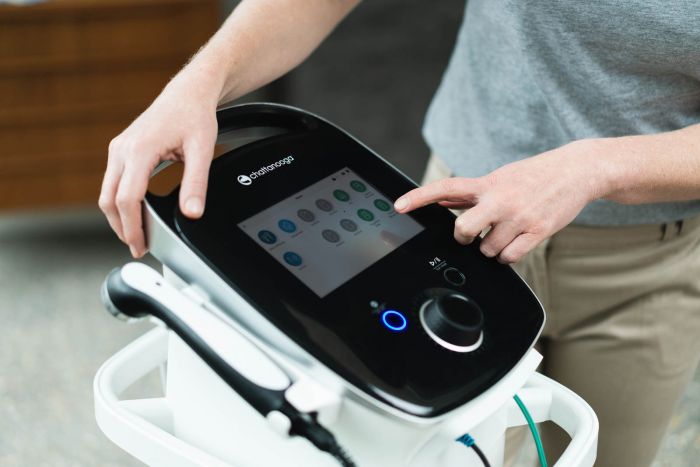
Intelect Mobile 2 COMBO
The Intelect Mobile 2 COMBO is a two-channel electrotherapy, ultrasound therapy and Combo system used with or without an optional Cart, allowing for the inclusion of a Vacuum module.
For combo therapy 5 waveforms are included for use: Interferential, VMS,High Volt, Asymmetric biphasic TENS and Symmetric biphasic TENS.
There is fully functional 1 and 3 MHz ultrasound frequencies and two channels of electrical stimulation output.
The intuitive user interface, quick access buttons and preset protocols means the Mobile 2 is both simple and effective for therapy delivery. The optional built in battery makes the Intelect Mobile 2 a truly mobile option.
KEY FEATURES
Full colour 7" high resolution touch screen
Upgradable software : future proof technology
Import / Export capability (Bluetooth)
Treatment data storage
Ergonomic design
Lightweight and mobile with ergonomically designed handle
Optional custom carry case available for safe and secure transport
Optional cart with storage drawers and large wheels
Home use: compliant with IEC 60601-1-11, can be used for treatment in the patients' home
KEY FEATURES - INTERFACE
Intelligent interface with touch buttons and interactive lights indicating status of device or port
Suggested Protocol Setting (SPS): built in suggested protocols based on currently available clinical evidence
Anatomical library with hi resolution images for patient explanation
Custom protocols can be stored
WAVEFORMS
Interferential current (Traditional IFC, 4-pole)
Premodulated IFC (2-pole)
Asymmetrical Biphasic current - TENS
Symmetrical Biphasic current - TENS
VMS
General
Main input voltages 100-240V AC, 1.0 to 0.42A, 50/60Hz
Electrical Class Class II
Mode of operation Continuous
Dimensions / Weight (no battery, excluding cart) 340 x 355 x 150 mm / 3.1 Kg
Conformity This device complies with the applicable standards EN/IEC 60601-1 and 60601-1-11, CAN/CSA-C22.2 No.601.1, UL Std. No 60601-1
CE 2797 Classification according to MDD : class IIa device 93/42 60601-1
Legal manufacturer DJO France SAS - CE 2797
Ultrasound
FREQUENCY 1MHz; 3 MHz
DUTY CYCLES 10%, 20%, 50%, Continuous
PULSE REPETITION RATE 16,48, or 100 Hz
PULSE DURATION
1-31.25 ms
- Max (ON): 31.25 ms
- Min (OFF): 5 ms
Ultrasound applicator
Ultrasound applicator Frequency 1cm² 2cm² 5cm²
1MHz 3MHz 1MHz 3MHz 1MHz 3MHz
Effective Radiating Area ERA INTL (cm²) 1 0.9 1.5 1 2.5 2.7
Max Output power in Continuous mode 2W 1.8W 3W 2W 5W 5.4W
Max Output power in Pulsed mode 3W 2.7W(*) 4.5W 3W 7.5W 8.1W
Max Amplitude in Continuous mode 2W/cm² 2W/cm² 2W/cm² 2W/cm² 2W/cm² 2W/cm²
Max Amplitude in Pulsed mode 3W/cm² 3W/cm² 3W/cm² 3W/cm² 3W/cm² 3W/cm²
Electrical Type (Degree of protection)
ULTRASOUND Type BF
ELECTROTHERAPY Type BF
ELECTROTHERAPY VACUUM Type BF
ELECTROTHERAPY & ULTRASOUND
Type BF
Vacuum
POWER INPUT 0-25 Vdc, maximum peak current 4A
ELECTRICAL TYPE Type BF
VACUUM RANGE 0 to 600 mbar maximum +/-5%
VACUUM MODES
Continuous or Pulsed Continuous
10 setting over vacuum range, 60 mbar per setting,
+10 mbar to 10 mbar per setting